Point-of-care relevance
Revolutionizing urine analysis at the point of care
Urine microscopy remains one of the most commonly performed tests in clinical labs, essential for screening and monitoring urinary tract infections, kidney diseases and other conditions. Manual microscopy is time-consuming and requires skilled personnel, while existing automated analyzers are often expensive and bulky.
The health fluidlab 1 uses cutting-edge holographic microscopy to perform automated urine particle analysis directly on uncentrifuged urine samples, eliminating the need for preparation steps and delivering fast, reliable results for point-of-care settings.
Designed for everyday use
Smart handling & operational guidance
Acella-Slide sample carrier
Ensures precise sample volume and consistent measurement conditions.
Factory-calibrated optics
Factory-calibrated, no-moving-parts sensor ensures long-term stability.
On-device storage & data transfer
Stores up to 300 measurements; data export via Wi-Fi or hotspot.
Automatic software updates
Regular enhancements delivered via Wi-Fi.
How it works
From light to insight
Digital holographic microscopy is an advanced imaging technique that captures detailed, quantitative phase information without the need for stains or dyes. This non-invasive method reconstructs high-resolution 3D images of cells and particles, while preserving their natural state.
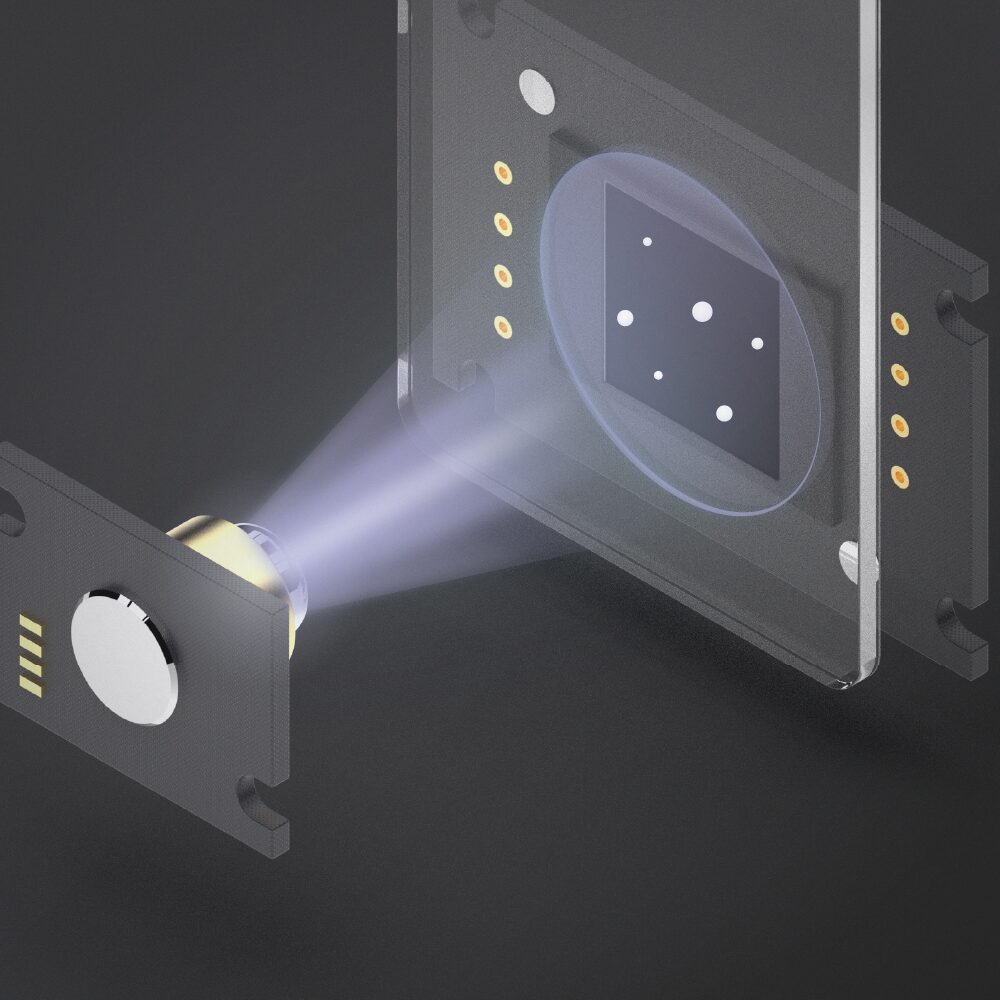
1. Illumination
A coherent light source illuminates the sample. Microscopic objects in the optical path scatter the light.
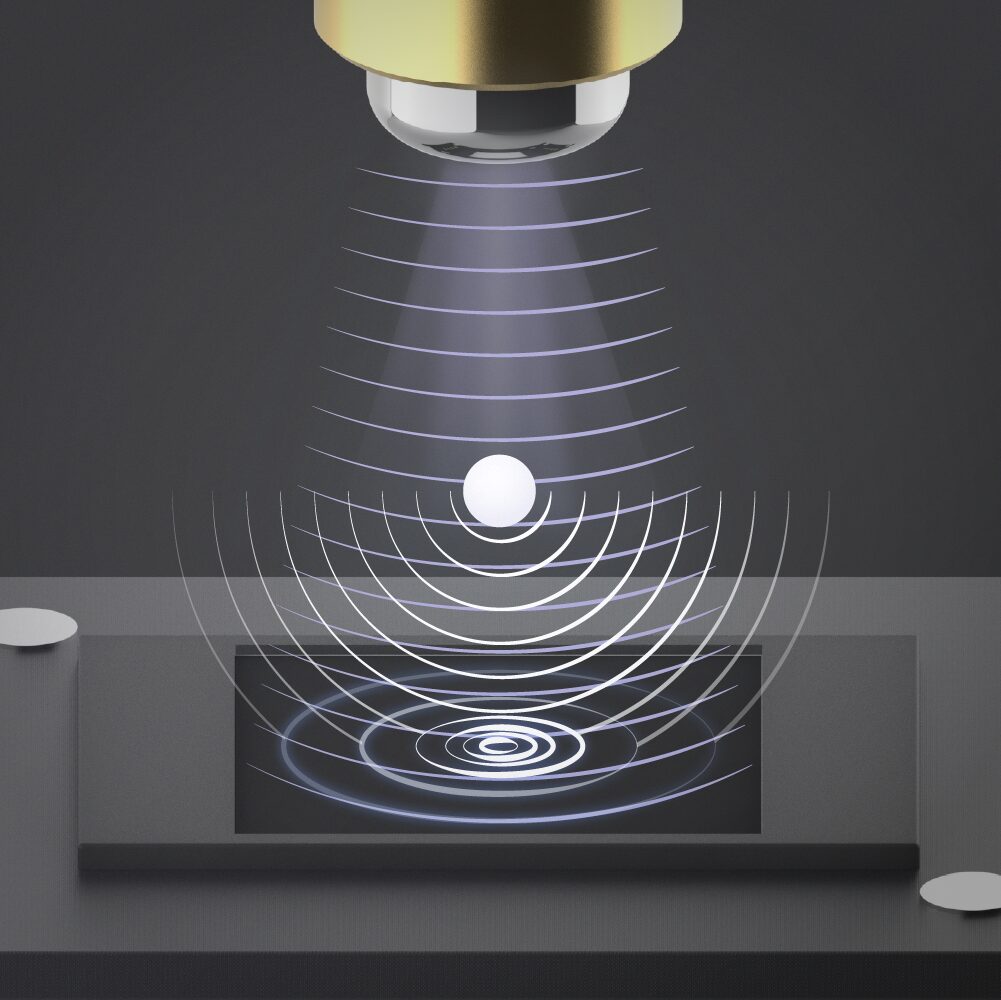
2. Hologram
The scattered light interacts with the original wavefront, forming an interference pattern – the hologram.
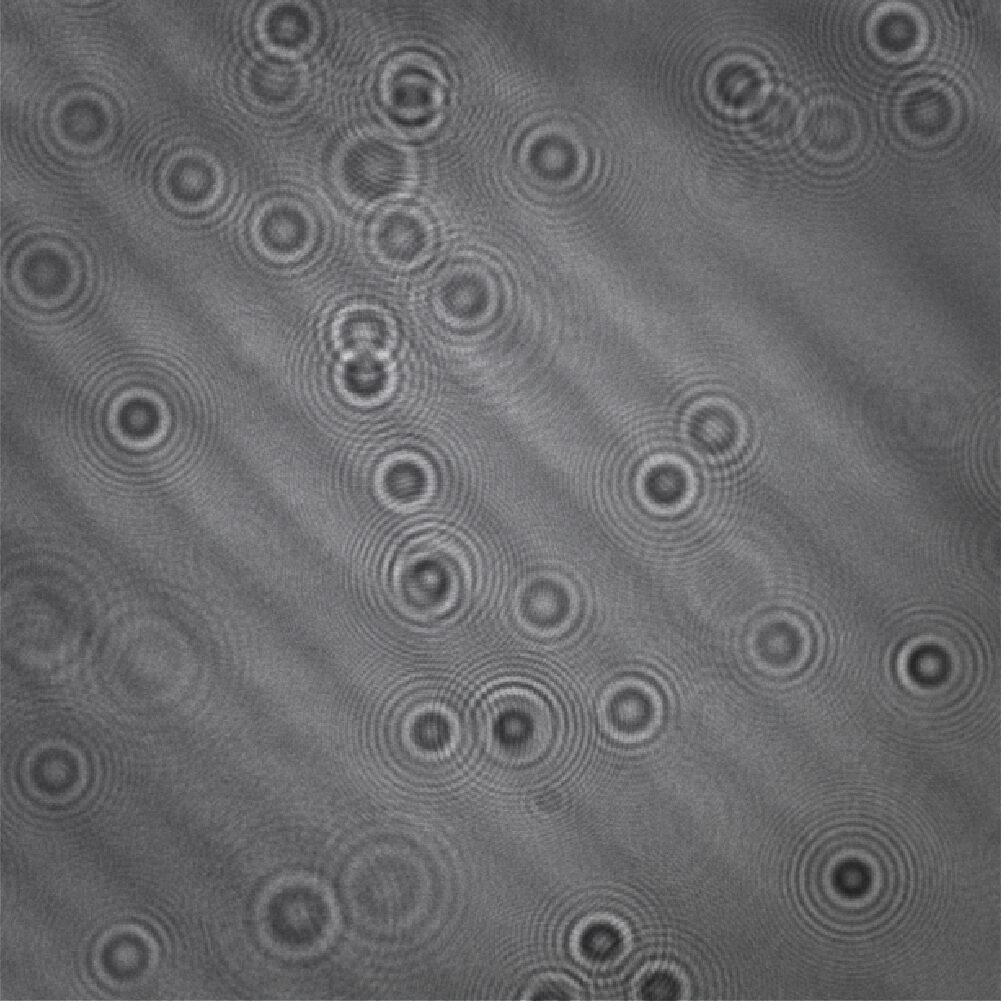
3. Reconstruction
Algorithms reconstruct phase and amplitudes to create quantitative 3D images.
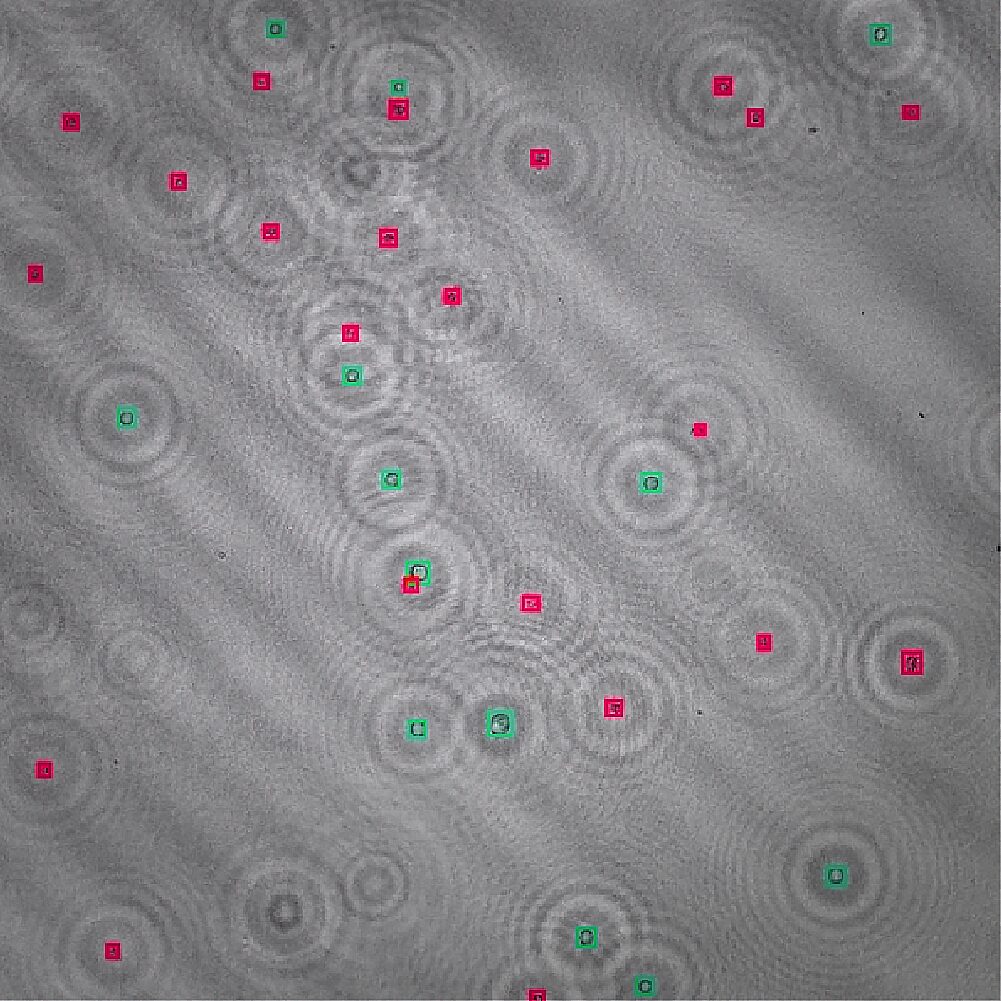
4. Classification
Machine learning models detect and classify cells and particles for the specific application.
expert perspective
Why point-of-care
diagnostics matter globally
Want to
learn more?
If you’re exploring point-of-care urine microscopy or considering the health fluidlab 1 for your workflow, our team is happy to support you.
Whether you need technical details, workflow guidance or want to discuss integration options — we’re here to help.

