Antibodies are proteins produced by B cells of the immune system in response to antigens and are therefore an important component of the immune system. Antigens are molecular structures that are recognized by the body as foreign and can bind to the antibodies. These can be, for example, proteins on cell surfaces of fungi, viruses or bacteria. As a result, the pathogens are recognized by the immune system and can be rendered harmless. Monoclonal antibodies are produced from only one cell clone, which originates from a single B lymphocyte. They are known to target a single epitope, which is why they have a particularly high specificity. Moreover, due to the possibility of continuous culture of hybridoma cells producing monoclonal antibodies, unlimited availability of the monoclonal antibodies can be guaranteed.
The development of monoclonal antibody production was a milestone for science and medicine, opening up new possibilities for cancer therapies, but also therapeutic agents for inflammatory diseases. In addition, they are used for detection and identification of serum analytes, cell markers and pathogens1.
Antibodies can thus be used in many different areas, which is why their production is of great importance for medicine and research. The fluidlab R-300, a combination of a spectrometer and an automatic cell counter, can support the production of monoclonal antibodies in the field of research and development.
Learn more in this article about
- the structure, shape, categories and function of antibodies in the immune response,
- passive and active immunization,
- the generation of monoclonal antibodies and how they differ from polyclonal antibodies,
- methods of antibody generation and the principle of production according to Milstein and Köhler,
- the importance of antibodies for research and
- the advantages of using the fluidlab R-300 for antibody production.
Test the fluidlab R-300 for the analysis of monoclonal antibodies free of charge and without obligation!
You want to analyze monoclonal antibodies and are interested in innovative technology that aims to do just that? Then test our patented fluidlab R-300 now - without any obligation!
Request test device now
Antibodies are key proteins for the body's immune response
Antibodies are effector molecules that are part of the humoral immune response. There are five classes of antibodies, which differ in size, shape or weight, among other things. Their main function is the labeling of pathogens (opsonization) or the neutralization of antigens.
Antibody structure, shape and categories
The five different classes of antibodies or immunoglobulins (Ig) have in common that they consist of two heavy and two light chains. The heavy chain bears a Greek letter as its name and also gives the name to the respective immunoglobulin. The five antibody classes are IgM, IgE, IgD, IgG and IgA. The two light polypeptide chains consist of 220 amino acids each and the two heavy ones of 450 amino acids each. The chains are linked by disulfide bridges (Fig. 1).
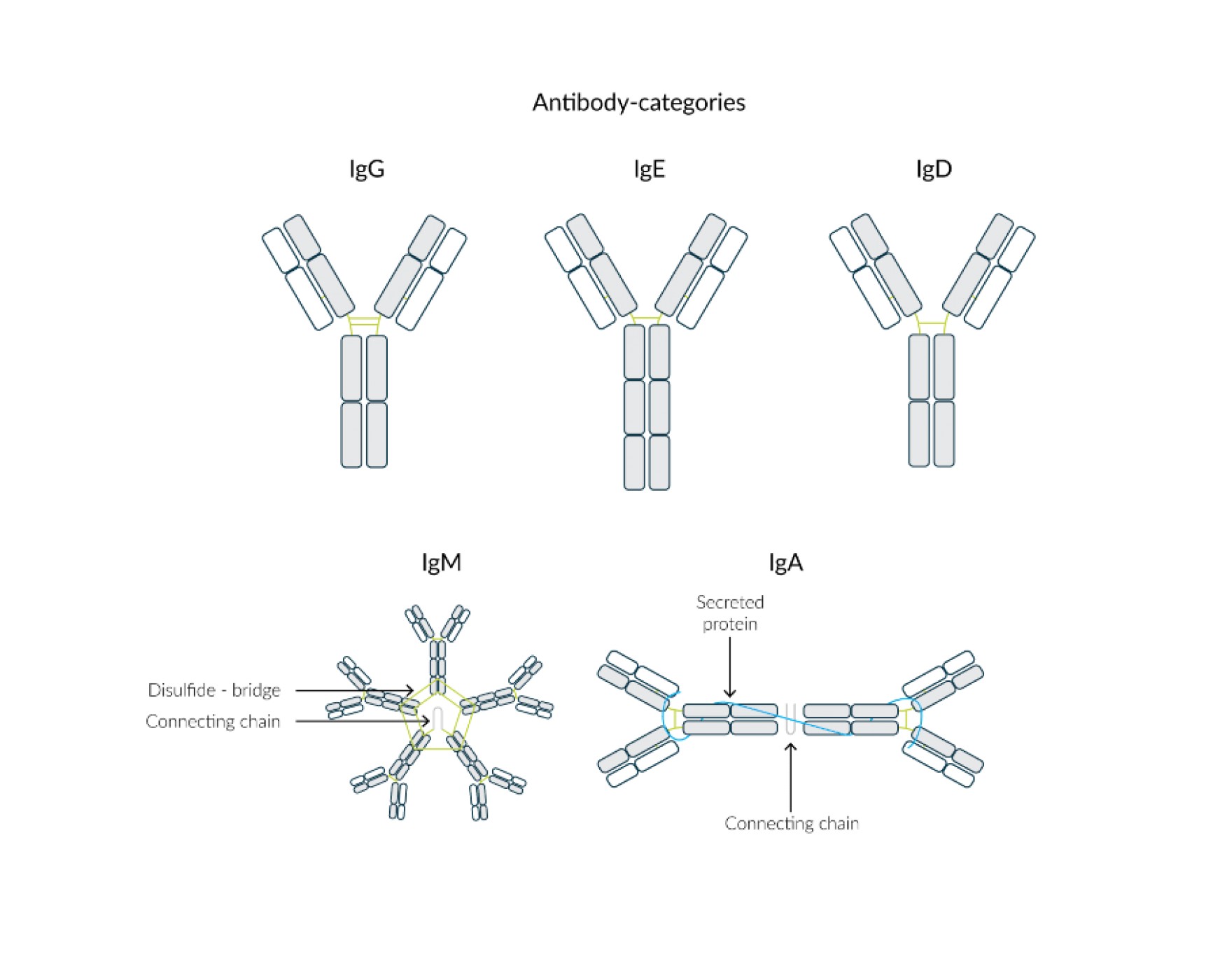
Fig. 1 Structure of the antibody classes IgG, IgE, IgD, IgM and IgA.
The inner region of the "Y" is the long, heavy chain, while the outer region of the two arms are the light chains. The initial part of the light chain as well as the heavy - i.e. the initial part of the Y-arms - is variable and crucial for the specificity of the antibody for different antigens. The Y-shape results from the bending of the heavy chains in the middle and is typical for immunoglobulin A, D, G and E (Fig. 1).
The IgM molecule differs visually from the others in that it consists of five basic units of two light and two heavy chains each, resulting in a pentamer and thus a five-rayed starfish. Within the IgA class, two units are able to join together to form a larger unit via a non-specific transport piece (Fig. 1). This makes them more resistant to fermentative degradation.2
The differences between the individual antibody classes are summarized in the following table (Tab. 1).
Tab. 1 Differences between the various antibody classes in molecular mass (kDA), plasma concentration (g/L), heavy chain, light chain, their occurrence in the organism and their respective main function.2
| Category | IgM | IgE | IgD | IgA | IgG |
|---|---|---|---|---|---|
| Molecular mass (kDa) | 900 | 200 | 180 | 150 (Dimer 400) | 150 |
| Plasma concentration (g/L) | 1,5 | 0,00005 | 0,03 | 3,5 | 13,5 |
| Heavy chain |
γ | ε | δ | α | γ |
| Light chain |
κ or λ | κ or λ | κ or λ | κ or λ | κ or λ |
| Occurence | surface of B-cells, blood serum | cell membrane of mast cells and granulocytes | surface of B-cells | mucous membranes, breast milk | Mucous membranes, blood serum |
| Main functions |
first defense against microorganisms in blood | parasite defense, affecting anaphylactic reaction | building B-cell receptor, affecting lymphocytes | mucosal protection, protecting newborns | protection extravascular space from bacteria and viruses, nest protection of newborns |
The function of antibodies in the immune response
The main function of antibodies is to recognize, label and neutralize pathogens. The antigens are labeled by specific binding of the antibodies (Fig. 2), which makes them recognizable for immune cells and the complement proteins for phagocytosis as well as for cytotoxic and lytic reactions. The individual functions of the different classes can in turn be differentiated in more detail (Tab. 1).
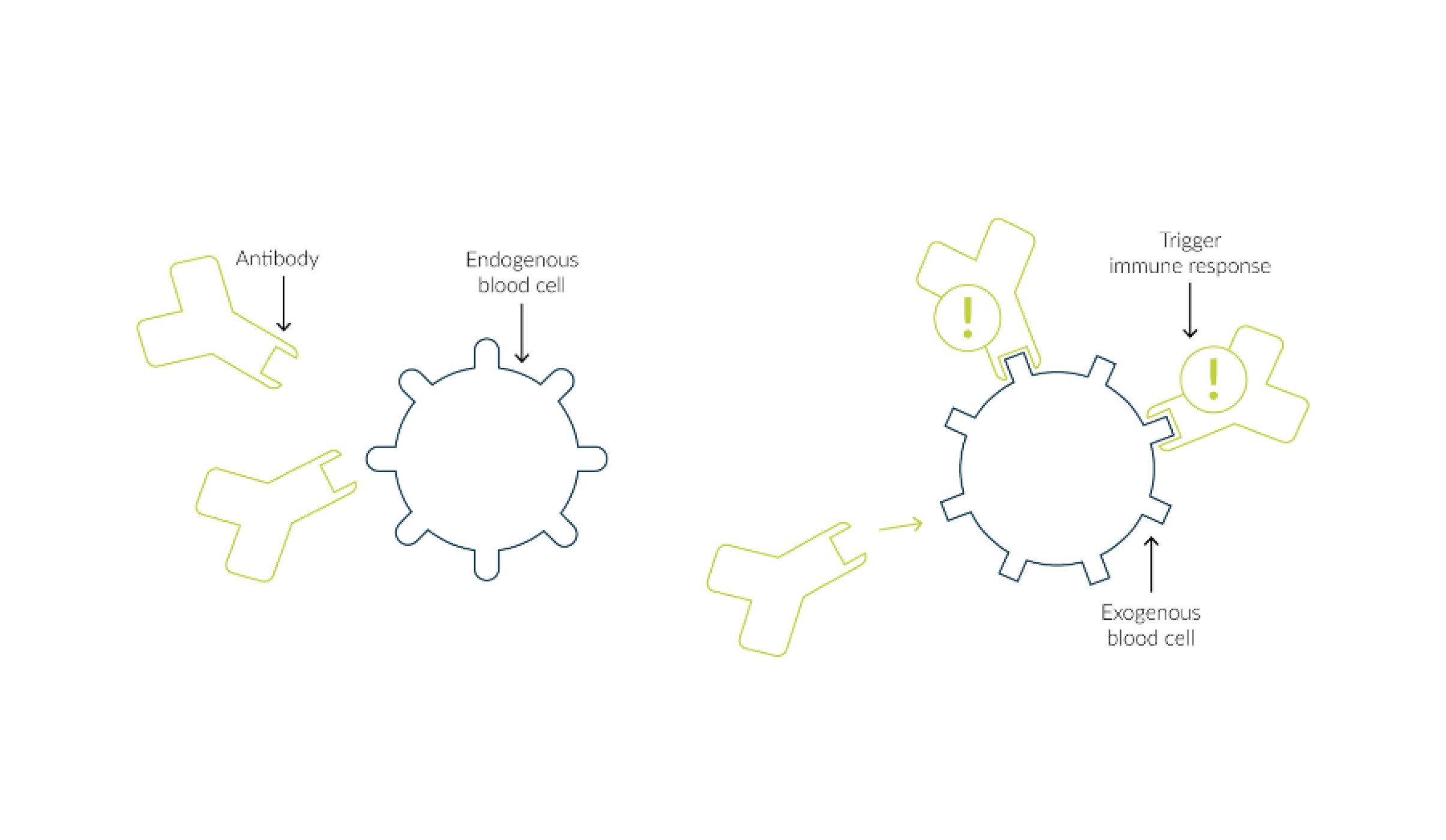
Fig. 2 Antibodies recognize a foreign blood cell, bind to it and can thus trigger an immune response.
IgM
IgM is predominant in the early phase of an infection and is mainly active against invading microorganisms. Production of the antibody usually occurs two to three days after initial contact with the antigen. Membrane-bound on B-cell surfaces, it takes over the function of an antigen receptor.
IgG
IgG, on the other hand, is important in repeated infection with the same antigen and is thus the most important antibody of the secondary immune response. This antibody occurs at the highest concentration in the body. Both IgM and IgG activate the complement system, a protein cascade of more than 20 components and regulators circulating in the blood, whose main function is the destruction and opsonization of pathogens and foreign bodies and the activation of defense cells. In addition, IgG binds to corresponding receptors on monocytes, macrophages and granulocytes, promoting phagocytosis of the bound antigen.
Außerdem handelt es sich bei IgG um den einzigen Antikörper, der die Plazentaschranke mittels rezeptorvermittelter Endozytose passieren kann. Dies hat zur Folge, dass die passive Immunität von der Mutter auf den Fötus übertragen wird. Zwei Tage nach dem ersten Auftreten von IgM erfolgt die Produktion von IgG.
IgA
IgA is the first line of defense in the local defense against infections and is mainly present in the digestive tract and in body fluids. The antibody prevents the attachment of viruses and bacteria to the surfaces of epithelia. IgA in the milk of a nursing mother additionally serves to protect the infant against the penetration of pathogens into the intestine.
IgD
IgD is present in the blood only in a very low concentration compared to the other antibody classes. It functions as an antigen receptor membrane-bound on B cells and is essential for the differentiation of B cells into plasma and memory B cells.
IgE
IgE is necessary for triggering allergic reactions and has a protective function against parasites.3
Passive vs. active immunization
Both passive and active immunization ensure that the organism becomes immune to certain pathogens. However, these two types of immunization differ from each other in several ways. In passive immunization, antibodies previously isolated from an already immune person are injected into another, non-immune person (Fig. 3).
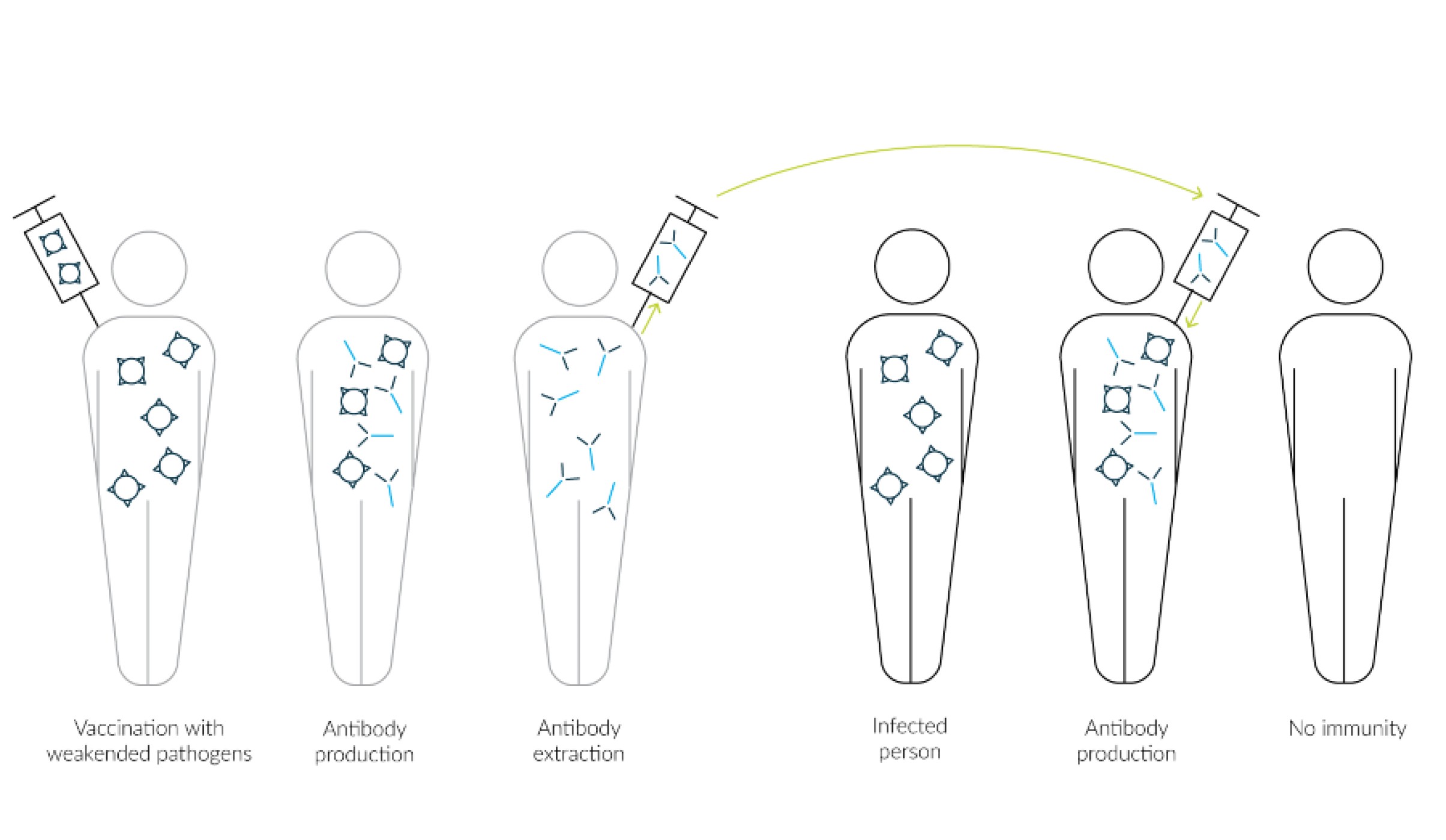
Fig. 3 Person 1 is actively immunized against an attenuated pathogen and forms antibodies. These are extracted and injected into a person infected with the pathogen for passive immunization. Subsequently, the person does not produce any antibodies of his or her own and thus no lasting immunity is achieved.
Passive immunization occurs when a non-immune person has already become infected with the pathogen and it is therefore too late for active immunization. The immune person from whom the antibodies are isolated may be someone who has already survived the infection with the pathogen and in the course of this has formed his or her own antibodies or has been actively immunized beforehand.
The advantage of passive immunization is that the injected antibodies are immediately effective and quickly put the pathogen out of action. However, these antibodies are broken down by the organism within a maximum of three months, as they were not produced by the organism itself and are therefore regarded as foreign bodies. For this reason, the vaccination protection lasts only for this time, because after passive immunization the body does not produce its own antibodies.
The advantage of passive immunization is that the antibodies are effective immediately after vaccination and quickly disable the pathogen. However, within a maximum of three months, these antibodies are broken down by the organism, since they were not produced by the organism itself and are therefore considered foreign bodies. For this reason, the vaccination protection lasts only for this time, because after passive immunization the body does not produce its own antibodies.
In contrast, active immunization involves the targeted infection of the organism with the attenuated pathogen, which results in the organism's own antibody production (Fig. 4).
The process of the body's own antibody production against a particular pathogen can take up to two weeks on average, so the antibodies are active much later in active immunization than in passive immunization. However, the actively formed antibodies can remain in the body for many years and are therefore still effective against the pathogen for a long time. The reason for this is the formation of B lymphocytes, also called memory cells. This results in the replication of the required antibodies in the event of a new infection with the pathogen. Accordingly, an antigen-specific immune memory is built up.
The pathogens in vaccinations are either attenuated or completely killed before administration. In addition, it happens that only characteristic features of the pathogen are vaccinated. Examples of active immunization include vaccines against tetanus, measles or mumps. 4
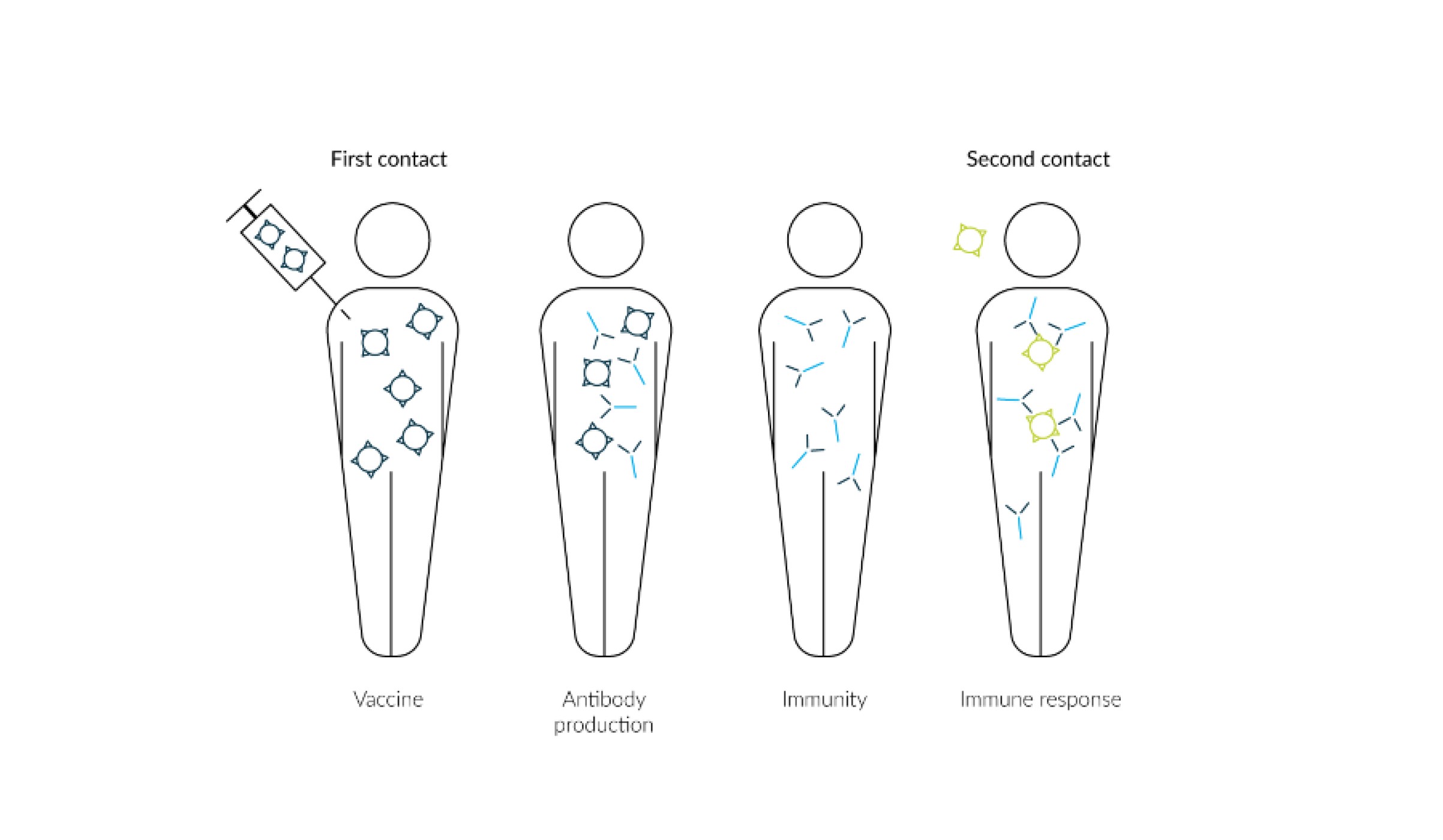
Fig. 4 A healthy person is actively immunized (vaccinated) by the injection of an attenuated pathogen. In the course of this, the person develops antibodies which circulate in the body for longer. This makes them immune to the pathogen, and an immune response can be triggered directly in the event of renewed contact.
The formation of monoclonal antibodies
In 1975, Köhler and Milstein developed a revolutionary method for the production of monoclonal antibodies. Both scientists were awarded the Nobel Prize for Medicine for this in 1984. 5
Monoclonal antibodies are directed against only one antigenic determinant of the material required for immunization. They are produced by a single B-lymphocyte clone only. The targeted production of monoclonal antibodies is achieved by the hybridoma technique. This is based on the culture and cloning of a single antibody-producing B lymphocyte. Thus, identical and thus monoclonal antibodies can be produced in large quantities.
Normally, B lymphocytes survive in culture for only a short time. For this reason, this technique fuses single antibody-producing B lymphocytes with cells from an unrestrictedly dividing myeloma. The B lymphocytes are derived from a mouse that is immunized against a specific antigen. In this way, hybrid cells, the so-called hybridoma cells, are created. Special selection media are then used to select these hybrid cells, which on the one hand produce the desired antibody and on the other hand can divide an infinite number of times. Each hybridoma cell thus gives rise to an individual cell clone, which grows without restriction and produces the specific (monoclonal) antibody.6
Monoclonal antibodies are mainly used as analytical reagents in diagnostics, research and therapeutic approaches.
The first functioning serum therapy that made use of antibodies was developed by Emil von Behring and Erich Wernicke in 1891. Together with Shibasaburo Kitasato, the researchers had isolated the tetanus-producing bacillus and deduced that the pathogenic effect was due to the release of a toxin. This was followed by a transfer of blood serum from a guinea pig immunized with diphtheria toxin to another guinea pig. As a result, the second guinea pig was found to be immune to the toxin. After numerous clinical tests, the first immunobiological therapeutic finally came onto the market three years later as an anti-diphtheria serum.7
Monoclonal vs. polyclonal antibodies
In contrast to monoclonal antibodies, the formation of polyclonal antibodies occurs during a naturally occurring immune reaction of the body. The resulting antibodies differ from monoclonal antibodies in many respects.
They are the product of B cells from several cell clones. They recognize different structures of the target antigen. In addition, their specificity and affinity may vary. After an individual has been immunized, purification of the polyclonal antibodies must take place before they can be used for immunization.
Monoclonal antibodies, on the other hand, as mentioned in the previous section, arise from hybridoma cells, a fusion of a B cell and a myeloma cell, which have been artificially cloned in the laboratory to form a specific cell line.
Therefore, the structure of monoclonal antibodies does not vary and they bind only one antigen at a specific site. Thus, they target a single antigenic determinant.
Compared to polyclonal antibodies, the use of monoclonal antibodies has clear advantages. For example, there are fewer cross-reactions of monoclonal antibodies, which increases their specificity, since they are directed against only one specific antigen at a time. Furthermore, they are produced in large quantities and are of consistent quality. In addition, monoclonal antibodies can be purified more easily than polyclonal antibodies.8
Method for the generation of monoclonal antibodies
There are various methods for generating monoclonal antibodies. Basically, it is a matter of getting cells to produce the desired antibodies. Milstein and Köhler were the first scientists to develop a method for producing monoclonal antibodies. They succeeded in doing so with the help of hybridoma cells.
Use of living organisms: The production principle according to Milstein and Köhler
The production of monoclonal antibodies according to Milstein and Köhler generally starts with the generation of hybrid cells by fusion of myeloma cells and antibody-producing B cells. In the manufacturing principle, the following steps are followed.
- Immunization of mice and isolation of antibody-producing splenocytes (here B cells)
After immunization of the mice with an antigen, screening of the blood is performed to check the production of antibodies. This is followed by isolation of antibody-producing splenocytes. - Preparation of myeloma cells
Myeloma cells are prepared for mixing with splenocytes to form a hybridoma with unlimited growth potential in the next step. - Fusion of myeloma cells and isolated splenocytes
This involves fusion of the myeloma cells with the isolated antibody-producing cells, resulting in the formation of hybridomas. Polyethylene glycol is used for this purpose, which creates a fusion of the cell membrane. - Screening and selection of clones
Given their antigen specificity and immunoglobulin class, screening as well as selection of clones is performed. - Functional characterization
Using an antibody-enzyme-linked immunosorbent assay (ELISA), highly secreting colonies are confirmed as well as validated and characterized. - Scale-up and weaning
In the penultimate step, clones producing the targeted antibodies are produced on a large scale and weaned from the selection medium. - Expansion
The last step is the expansion of the clones that produce the desired antibodies, for example in bioreactors.6
The use of vectors for the production of monoclonal antibodies
In the meantime, apart from the hybridoma technique, there are also other methods for producing monoclonal antibodies, especially on a large scale. Vectors containing the target DNA are produced in the first production step. Glutamine synthetase (GS) gene expression systems and those based on dihydrofolate reductase (DHFR) are mostly used to produce therapeutic monoclonal antibodies.
The vectors produced are propagated in bacteria, such as Escherichia coli, and then transfected into cells. These are usually CHO, HEK, Sf9 or HeLa cells. However, yeast cells are also used in some expression systems. This is followed by screening of the cells to select the successfully transfected cells that produce the antibodies. Finally, scale-up and expansion into large bioreactors takes place, where the antibodies are produced on a large scale using the fed-batch method.9
Alternative in vitro technologies for monoclonal antibody production
However, monoclonal antibodies can also be produced in ways other than those previously described. One possibility is phage display10, which involves, among other things, presenting certain structures such as antigens on cell surfaces and using them in antibody banks. These banks contain many different gene segments for antibodies. Each phage contains a different sequence for antibodies.
Fig. 5 The target protein is incorporated into the phage envelope. Upon presentation of the appropriate ligand, phages with the target protein can bind to it and thus be selected.
In a phage display, the first step is the isolation of plasma cells from the organism, from which the mRNA is isolated and transcribed into cDNA. The genes contained in the cDNA are then amplified via a polymerase chain reaction (PCR).
Integration of the gene segments into a phagemid vector and subsequent transfer into E.coli, results in the expression of fusion proteins, which are transported into the periplasm and modified into coat proteins.
The resulting proteins are incorporated into the phage envelope during new formation, where they perform the function of a binding site for antigen ligands (Fig. 5). The phages can be isolated by introducing specific ligands to which the phages bind with the target proteins. These steps can be repeated until the target protein is present at the desired concentration.11
The importance of monoclonal antibodies for research
The use of monoclonal antibodies is very diverse, which makes them extremely important for research. Depending on their specific effect, they can therefore be used in different ways. While some are directed against antimicrobial peptides, so-called soluble factors12 , and have the task of neutralizing them, there are others which are directed against receptors. The resulting blockage of the receptor has the effect of inhibiting the signaling cascade induced by the natural ligand.
Use in medicine
Antibodies are also used in the development of active ingredients and pharmaceuticals. One example is the active ingredient Nivolumab, which is a monoclonal antibody that can be used specifically against advanced non-small-cell bronchial cancer and thus leads to a strong improvement in the health of patients.
Furthermore, they are used to develop drugs that can bind messenger substances.
The drug Infliximab, for example, binds a messenger substance called TNF-alpha, thereby neutralizing its biological effect as an inflammatory factor. For this reason, it is used in the treatment of various inflammatory diseases such as Crohn's disease, rheumatoid arthritis, ankylosing spondylitis, psoriasis or even ulcerative colitis.
The active ingredient bevacizumab is also used to treat non-small cell lung cancer. Its effect lies in the binding of vascular endothelial growth factor (VEGF), which acts as a messenger in angiogenesis. During tumor angiogenesis, messenger substances are sent by the carcinoma that promote angiogenesis, the formation of new blood vessels, in the direction of the tumor, thus enabling it to connect to the vascular system to grow.13
Use in diagnostics
Antibodies can play a decisive role in the diagnosis and therapy of tumor diseases. In pathological diagnostics, a very precise diagnosis can be made with the aid of a histological image, clinical information and a medical history using a few, but highly specific antibodies from a diseased person.
The procedure is usually performed on a kerosene section with as much vital and representative tumor tissue as possible. After appropriate preparation of the tissue, labeling with a suitable antibody is performed. First, a primary antibody is used, which binds specifically to the intended binding site in the tissue. This is followed by the addition of a secondary antibody, which is coupled to a polymer and binds to the primary antibody. The second antibody, or the coupling with the polymer, allows the antibodies to be detected. With the help of the matching antibodies, the entity and subtype of a tumor can be determined. In the case of a metastasis, the containment of a primary tumor can be determined. For example, when detecting a tumor in the lung, it is possible to distinguish between the subtypes adenocarcinoma and squamous cell carcinoma by having the corresponding antibodies bind to CK7 or TTF1 - for the former - or to CK5/6 or p40, which would indicate squamous cell carcinoma. 14
Study on improved cancer therapy with antibodies
Research with antibodies in cancer therapy has been ongoing for several years. In the available immunotherapy treatment options, the body's own immune system is recruited to destroy tumor tissue by the added antibody, CD40, binding to surfaces of immune cells, activating them and stimulating the production of the body's own killer T cells. In clinical trials, less than 20% of patients responded to this treatment. The observation here was that the T-killer cells produced could not reach the inside of the tumor because the vascular system of tumors is leaky and underdeveloped.
The 2019 study15 combined three different antibodies that fulfilled two different mechanisms of action, resulting in more effective tumor destruction. This resulted in the addition of two other antibodies to CD40 treatment, which have anti-angiogenic effects and were responsible for stabilizing tumor blood vessels. The use of this antibody combination in various animal models with different types of cancer showed that this led to a significantly greater destruction of the tumor tissue compared to the results obtained previously. The next step will be clinical studies in humans.15
Use of antibodies in laboratory analyses
In the laboratory, antibodies can be analyzed by immunological tests, for example with an ELISA. This method is based on an enzymatic color reaction. Initially, the antigen to be detected binds to a microtiter plate and thus accumulates via an initial antibody. An enzyme-coupled second antibody leads to the color reaction or chemiluminescence (Fig. 6). Finally, a photometer can be used to measure the signal strength, which provides information about the concentration of the antibody within a sample/sorbent.16
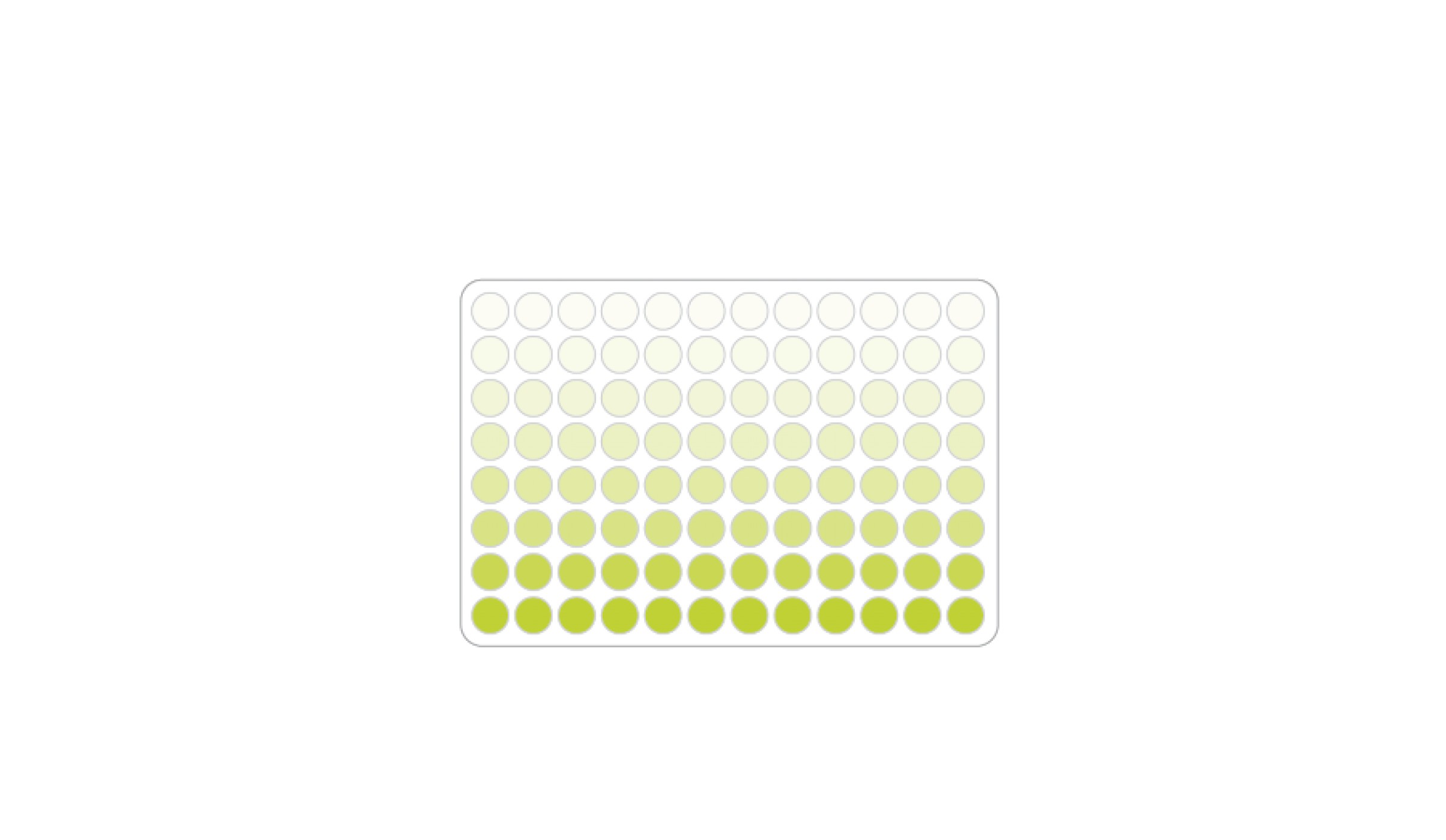
Fig. 6 Microtiter plate with different antibody concentrations. Color gradient results from an enzymatic color reaction depending on the antibody concentration.
Immunological tests also include rapid tests, whereby the antibodies are applied to a paper strip or other carrier. The liquids that can be used for the rapid tests are, for example, saliva, urine or blood. The tests are easy to use and provide a quick result.
After application of the liquid, it migrates along the paper strip from one side to the other (Fig. 7). If the sample contains the desired substance, represented by the corresponding antigen, this is bound to the antibodies on the test strip and a color reaction occurs. In most cases, a red line is visible. If the liquid reaches the end of the test strip, a red line (green in Fig. 7) is also visible in the control window, which means that there was sufficient liquid on the carrier and the test has run correctly. Such procedures are mainly known from pregnancy tests or from COVID-19 rapid tests.17
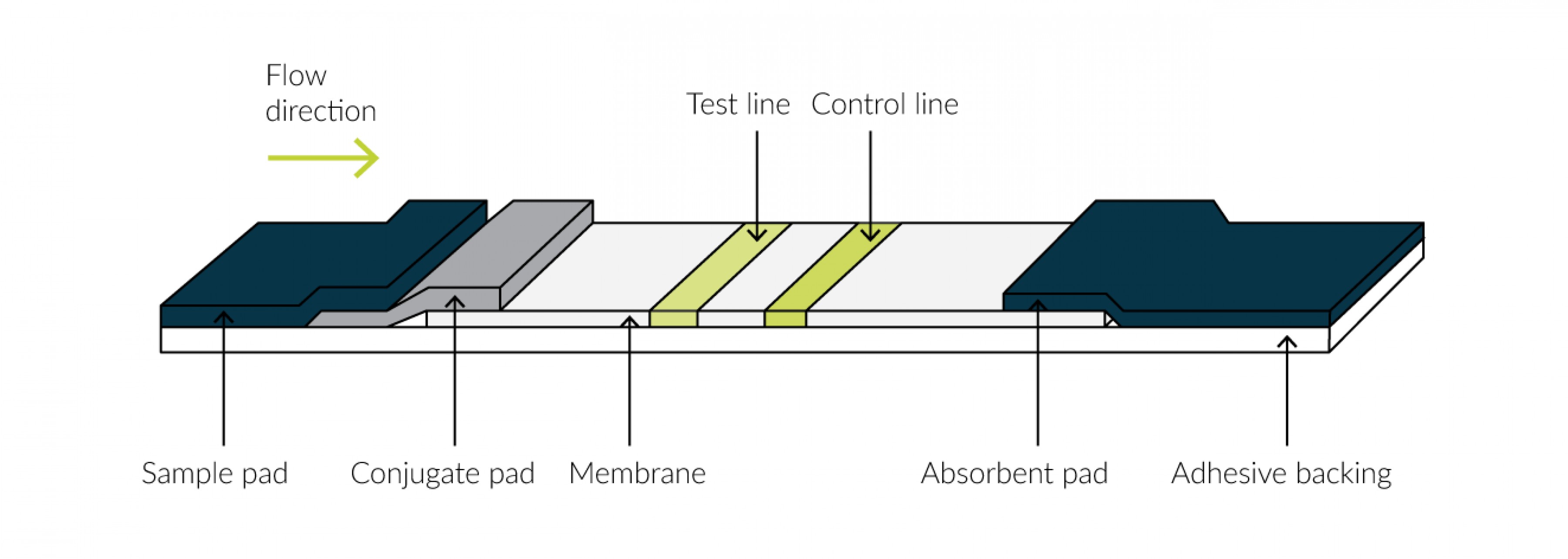
Fig. 7 Carrier with membrane. Liquid is applied to the test pad and runs along the membrane according to the flow direction. Antibodies are present on the test line, leading to visualization of the line when the antigen from the applied liquid binds to it. The control line becomes visible when sufficient liquid has reached it.
The advantages of using the fluidlabs R-300 in antibody production steps
The fluidlab R-300 is a small analyzer that combines an innovative cell counter and a powerful photospectrometer in one. Its small size, which allows it to fit in the palm of your hand, and its light weight make it easy to transport. These features also bring the advantage that measurements with the fluidlab can be performed directly under the sterile workbench.
Viability is measured without the use of dyes and the holographic microscope does not need to be calibrated. This saves time and the accuracy of the measurement is increased by omitting the cytotoxic dye, trypan blue.
In antibody production, the fluidlab R-300 can be used in several steps of protein production.
When preparing the vectors, during bacterial growth, the spectrometer of the fluidlabs OD₆₀₀ can be used to perform measurements that provide information about the bacterial quantity. This plays an important role in antibody production, as the bacteria contain vectors to be able to form the antibodies later in cells (see above procedure for generating monoclonal antibodies).

Fig. 8 The fluidlab R-300 can be used in various steps of antibody production. During the preparation of the vectors and their propagation in bacteria, the fluidlab can be used to measure the OD600. After introduction of the vectors into cells, the cell counter of the R-300 can be used to count the cells and determine their viability without staining. For protein determination, the photospectrometer of the fluidlab measures the absorbance and the concentration of the proteins can be determined directly and easily using a created calibration curve.
The cells in which the antibodies are formed can thus be automatically counted with the fluidlab and their viability determined without staining. The large field of view of 5.3 mm² and the fully automated measurement generate exact, reproducible results and a high degree of statistical certainty. Typical cells used in protein determination have already been validated for measurement with the fluidlab R-300:
- HEK
- CHO
- HeLa
- Sf9
In the final step of antibody production, the antibodies must be extracted, purified and quantified. The latter is usually done by Bradford protein determination at 595 nm and can therefore also be performed with the fluidlab. Here, the photospectrometer of the fluidlab offers the possibility of creating and storing calibration curves for the rapid measurement of the colorimetric assays.
In addition, anvajo's free data export software offers simultaneous transfer of results to any number of end devices, making data transfer and analysis even easier.
Test the fluidlab R-300 free of charge and without obligation!
Request test device now
Sources
1 Nelson, P. N., Reynolds, G. M., Waldron, E. E., Ward, E., Giannopoulos, K., & Murray, P. G. (2000). Monoclonal antibodies. Molecular pathology: MP, 53(3), 111–117.
2 Oswin G. (1971). Grundlagen der Immunologie:II. Antikörperstruktur und -funktion; Spätreaktion, Deutsches Ärzteblatt, 68(26): A-1983.
3 Edelmann G.M. (1970). The structure and function of antibodies. Scientific American, (223): 34-42.
4 Bröker B. et al. (2019). Immunisierung. In: Grundwissen Immunologie, 4 (13): 247-254.
5 Mössner, J., Neubauer, A. (2019). Monoklonale Antikörper. Internist, 60: 1009–1013.
6 Köhler, G., Milstein, C. (1975). Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256: 495–497.
7 Yamada T. (2011). Therapeutic monoclonal antibodies. The Keio journal of medicine, 60(2): 37–46.
8 Janeway CA et al. (2001). Immunobiology. The Immune System in Health and Disease, (5): 9-12 ff.
9 Birch, J. R., & Racher, A. J. (2006). Antibody production. Advanced drug delivery reviews, 58(5-6), 671–685.
10 Smith G. P. (2019). Phage Display: Simple Evolution in a Petri Dish (Nobel Lecture). Angewandte Chemie, 58 (41): 14428-14437.
11 Jonas, G. et al. (2001). Binding of phage-displayed HIV-1 Tat to TAR RNA in the presence of cyclin T1. Journal of biomedical science, 8(5): 430–436.
12 Rink L. et al., (2012). Das angeborene Immunsystem. In: Immunologie für Einsteiger. Spektrum Akademischer Verlag, 1(30): 39.
13 Klebe, G. (2019). Entwurf und Wirkung von Arzneistoffen. Wirkstoffdesign. Spektrum Akademischer Verlag, (2): 634.
14 Förster, S., & Tannapfel, A. (2019). Einsatz monoklonaler Antikörper in der pathologischen Diagnostik. Use of monoclonal antibodies in pathological diagnostics. Der Internist, 60(10): 1021–1031.
15 Abhishek S. A. et al. (2019). Optimized antiangiogenic reprogramming of the tumor microenvironment potentiates CD40 immunotherapy. Proceedings oft he National Academy of Sciences of the United States of America, 117 (1): 541-551.
16 Lequin R. M. (2005). Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clinical chemistry, 51(12): 2415–2418.
17 Neumeister B et al. (2018). Arbeitsmethoden, Referenzbereiche, Differentialdiagnose, Diagnosestrategien. Klinikleitfaden Labordiagnostik, Stuttgart: Urban und Fischer, (6): 265.






